How To Say Tetraborate
adminse
Apr 07, 2025 · 7 min read
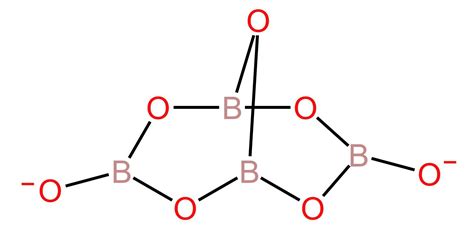
Table of Contents
How to Say Tetraborate: A Deep Dive into Naming and Understanding Borates
What makes the naming of tetraborate so complex and fascinating?
Understanding the nomenclature of tetraborate unlocks a deeper understanding of its chemical properties and applications.
Editor’s Note: This comprehensive guide to understanding and articulating the name "tetraborate" has been published today.
Why "Tetraborate" Matters
The seemingly simple term "tetraborate" actually represents a family of chemical compounds, primarily encompassing various salts of boric acid. Understanding how to correctly name and identify these compounds is crucial across multiple disciplines. From chemistry and materials science to medicine and agriculture, borates play vital roles, demanding precise communication about their specific composition. Incorrect naming can lead to confusion, hindering research, manufacturing processes, and even safety protocols. This article provides a detailed exploration of the nomenclature surrounding tetraborates, clarifying the intricacies of its naming conventions and explaining the significance of precise terminology.
Overview of the Article
This article will dissect the term "tetraborate," exploring its origins, the different forms it takes, and the nuances in its naming conventions. We will examine the underlying chemistry, the various applications of different tetraborate compounds, and discuss common misconceptions. Readers will gain a comprehensive understanding of the complexities surrounding this seemingly simple term, allowing for more accurate communication and a deeper appreciation of borate chemistry.
Research and Effort Behind the Insights
The information presented here is based on extensive research drawn from peer-reviewed scientific literature, chemical handbooks, and reputable online resources. The article synthesizes this information to provide a clear and concise explanation of the topic, avoiding technical jargon where possible while maintaining scientific accuracy.
Key Takeaways
| Key Point | Explanation |
|---|---|
| Tetraborate is not a single compound | It refers to a family of salts derived from boric acid. |
| Anion Variation is Key | The key to naming lies in understanding the specific borate anion present (e.g., tetraborate, metaborate). |
| Cation Influences the Name | The cation (positive ion) associated with the borate anion significantly influences the compound's complete name. |
| Hydration States Matter | The number of water molecules associated with the salt also affects its name and properties. |
| Systematic vs. Common Names | Both systematic (IUPAC) and common names exist, leading to potential ambiguity. |
Smooth Transition to Core Discussion
Let’s delve into the core aspects of tetraborate nomenclature, beginning with an exploration of the boric acid structure and its subsequent salt formation.
Exploring the Key Aspects of Tetraborate Naming
-
Boric Acid as the Precursor: Tetraborates are derived from boric acid (H₃BO₃). Understanding the structure of boric acid is fundamental. It's a weak acid that readily forms various anions depending on pH and reaction conditions.
-
The Tetraborate Anion (B₄O₇²⁻): The most common borate anion is the tetraborate ion, B₄O₇²⁻. This complex ion is formed through the condensation of boric acid molecules, resulting in a cyclic structure. The "tetra" prefix indicates the presence of four boron atoms in the anion.
-
Cationic Variation: The tetraborate anion combines with various cations (positive ions) to form different tetraborate salts. Common cations include sodium (Na⁺), potassium (K⁺), and calcium (Ca²⁺). The cation significantly influences the overall properties and name of the resulting compound. For instance, sodium tetraborate is vastly different from calcium tetraborate in its solubility and applications.
-
Hydration States: Many tetraborate salts exist in hydrated forms, meaning they incorporate water molecules into their crystal structures. The number of water molecules significantly affects the compound's physical properties. For example, sodium tetraborate decahydrate (Na₂B₄O₇·10H₂O), also known as borax, is vastly different from anhydrous sodium tetraborate (Na₂B₄O₇).
-
Systematic vs. Common Names: The chemical nomenclature can be confusing due to the use of both systematic (IUPAC) and common names. The systematic name clearly reflects the chemical composition, while common names are often based on historical usage or traditional practices. This duality can lead to ambiguity, especially for those unfamiliar with borate chemistry. For instance, borax is the common name for sodium tetraborate decahydrate.
Closing Insights
The term "tetraborate" is far from simple, representing a broad family of compounds with varying compositions and applications. Understanding the nuances of borate nomenclature, considering the cation, the presence of hydration water, and the choice between systematic and common naming, is essential for accurate communication and effective application in various fields. Precision in terminology ensures clarity and minimizes potential errors in scientific research, industrial processes, and practical applications.
Exploring the Connection Between Hydration and Tetraborate Properties
The number of water molecules associated with a tetraborate salt dramatically influences its properties. For example, borax (sodium tetraborate decahydrate) is a readily soluble white crystalline powder, while anhydrous sodium tetraborate is less soluble and possesses different crystal structures. This difference in solubility affects its usage in various applications, from laundry detergents (borax) to specialized chemical processes (anhydrous sodium tetraborate).
Further Analysis of Hydration
| Hydration State | Formula | Solubility in Water | Common Name | Applications |
|---|---|---|---|---|
| Anhydrous | Na₂B₄O₇ | Low | Anhydrous Borax | Glass manufacturing, ceramic glazes |
| Pentahydrate | Na₂B₄O₇·5H₂O | Moderate | Sodium Tetraborate Pentahydrate | Certain industrial applications, less common than decahydrate |
| Decahydrate | Na₂B₄O₇·10H₂O | High | Borax | Detergents, cleaning agents, fertilizers |
FAQ Section
-
What is the difference between borax and sodium tetraborate? Borax is the common name for sodium tetraborate decahydrate (Na₂B₄O₇·10H₂O). They are essentially the same compound, but the common name is more widely used in commercial contexts.
-
How can I identify a specific tetraborate compound? Accurate identification requires chemical analysis techniques such as titration, spectroscopy, or X-ray diffraction. The specific cation and the level of hydration can be determined through these methods.
-
Are all tetraborates equally safe? The toxicity of tetraborates varies depending on the specific compound and the level of exposure. Borax, for example, is generally considered safe for household use in low concentrations, but higher concentrations or ingestion can be harmful.
-
What are the industrial applications of tetraborates? Tetraborates are extensively used in glass manufacturing, ceramics, detergents, pesticides, and as a flame retardant. The specific application depends on the properties of the individual tetraborate compound.
-
Where can I buy tetraborate compounds? Tetraborate compounds are available from chemical suppliers, online retailers, and some hardware stores (particularly borax). Always ensure you purchase from reputable sources to guarantee purity and safety.
-
What are the environmental considerations associated with tetraborates? While generally considered low toxicity, large-scale use of tetraborates can have environmental impacts. Proper disposal and sustainable practices are crucial to minimize environmental consequences.
Practical Tips
-
Always specify the cation and hydration state when discussing tetraborates. Avoid ambiguity by using precise chemical formulas or names.
-
Consult safety data sheets (SDS) before handling any tetraborate compound. SDSs provide crucial information regarding safety precautions, hazards, and proper handling procedures.
-
Use appropriate personal protective equipment (PPE) when working with tetraborates. PPE can include gloves, eye protection, and respirators depending on the specific compound and the nature of the work.
-
Store tetraborate compounds appropriately. Many tetraborates are hygroscopic (absorb moisture from the air), so they should be stored in airtight containers in a dry environment.
-
Understand the different applications of various tetraborate compounds. Choose the appropriate tetraborate for your specific needs based on its properties and intended use.
-
Dispose of tetraborates responsibly. Follow local regulations for the proper disposal of chemical waste to minimize environmental impact.
-
When purchasing tetraborates, verify purity and source. Ensure the supplier is reputable and provides accurate information about the product's composition.
-
If working with tetraborates in a professional setting, adhere to all relevant safety regulations and guidelines. This includes proper ventilation, waste disposal, and emergency procedures.
Final Conclusion
The seemingly simple term "tetraborate" hides a complex world of chemical intricacies. Precise naming conventions are essential for clarity and safety. This exploration has uncovered the importance of specifying cation, hydration level, and whether using systematic or common names. By understanding these nuances, we can effectively communicate about and utilize the wide range of tetraborate compounds in various fields, furthering research, innovation, and responsible application. The complexities of tetraborate nomenclature underscore the need for precise language in science and industry. Continued exploration of borate chemistry promises further discoveries and technological advancements.
Latest Posts
Latest Posts
-
How To Say Tastes Good In German
Apr 08, 2025
-
How To Say Brother In Plains Cree
Apr 08, 2025
-
How To Say Please In Khmer
Apr 08, 2025
-
How To Say Garbage Man In Spanish
Apr 08, 2025
-
How To Say Strudel In German
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about How To Say Tetraborate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.